| |
| |
Effects of nandrolone
decanoate compared with placebo
or
testosterone on HIV-associated wasting. |
| |
| |
HIV Medicine
Volume 7 Page 146 - April 2006
J Gold1, MJ
Batterham2, H Rekers3, MK Harms3, TBP Geurts3, PME
Helmyr3, J Silva de Mendonca4, LH Falleiros
Carvalho5, G Panos6, A Pinchera7, F Aiuti8, C
Lee9, A Horban10, J Gatell11, P Phanuphak12, W
Prasithsirikul13, B Gazzard14, M Bloch15 and SA
Danner16 for the E-1696 Study Investigators
"...This study is
the first large multicentre randomized
placebo-controlled study to demonstrate the
effectiveness of nandrolone decanoate over placebo
in increasing fat free mass, weight and body mass
index in HIV-positive males with wasting. In
addition, fortnightly treatment with 150 mg
nandrolone decanoate was superior to fortnightly
treatment with 250 mg testosterone for increasing
weight, increasing body mass index and
(insignificantly) increasing fat-free mass. Subjectively, nandrolone was superior to both
placebo and testosterone for improving perception
of treatment benefit and recovery from
symptoms..."
Table 3 Summary statistics of changes
from baseline to endpoint during the initial
treatment period in anorexia/cachexia results
(intention-to-treat analysis)
"The use of this instrument in the
present study clearly demonstrated a significant
benefit of nandrolone over both placebo and
testosterone for subjective improvements in
recovery of symptoms from anorexia/cachexia and
patient perception of benefit. This questionnaire
was designed to target the aspects of quality of
life that are specific to a nutritional/appetite
intervention, such as body image, enjoyment of
food, appetite per se and the actual intervention.
These areas are not covered in traditional quality
of life questionnaires and therefore a treatment
effect may be missed."
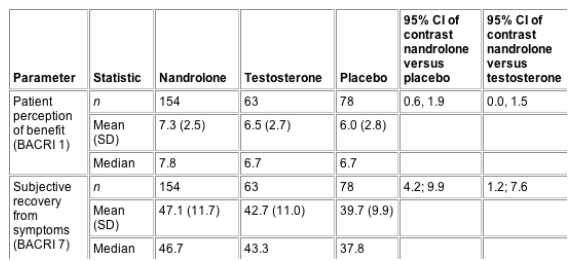
BACRI, Bristol-Myers Anorexia
Cachexia Recovery Instrument; CI, confidence
interval; SD, standard deviation.
ABSTRACT
Objectives
Current
research is unclear about the most effective
pharmacological agents for managing the loss of
weight and fat-free mass common in HIV/AIDS. The
aim of this study was to compare nandrolone
decanoate with placebo and testosterone.
Methods
The study was a multicentre randomized
double-blind placebo-controlled trial. Three
hundred and three adult HIV-positive male patients
with a weight loss of 5-15% in the last 12 months,
or a body mass index of 17-19 kg/m2, or a body
cell mass/height ratio lower than 13.5 kg/m, were
randomly assigned to receive nandrolone decanoate
(150 mg), testosterone (250 mg) or placebo
intramuscularly every 2 weeks for 12 weeks.
Fat-free mass, weight, immune markers and
perception of treatment were the main outcome
measures.
Results
Treatment
with nandrolone resulted in significantly greater
increases in fat-free mass [mean increase 1.34 kg;
95% confidence interval (CI) 0.60; 2.08 kg] and in
weight (mean increase 1.48 kg; 95% CI 0.82; 2.14
kg) compared with placebo. The mean increase in
weight with nandrolone of 1.00 kg (95% CI 0.27;
1.74 kg) when compared with testosterone was
significant, although the difference in fat free
mass did not reach significance (mean increase
0.69 kg; 95% CI-0.13; 1.51 kg). Patient perception
of benefit was significantly greater in the
nandrolone group when compared with both the
placebo and the testosterone groups.
Conclusions
Treatment with nandrolone decanoate
increased body weight when compared with placebo
and testosterone. Nandrolone decanoate treatment
resulted in greater increases in fat-free mass
than placebo and demonstrated a trend for a
significant increase when compared with
testosterone.
LIMITATIONS
The
results reported in this study are relatively
short-term reporting changes over a 12-week
period. Grinspoon et al [34] have conducted a
long-term study of the use of testosterone showing
sustained significant increases in fat-free mass
and smaller changes in weight. However, the
long-term use of nandrolone requires
investigation, as clinically patients are using
these treatments long-term where the efficacy has
not yet been demonstrated.
Clinically, this study demonstrates
unequivocally the superiority of nandrolone
decanoate over placebo for increasing fat-free
mass, weight, body mass index, and perception of
treatment benefit when compared with placebo. In
addition, nandrolone was superior to testosterone
for increasing weight and perception of treatment
benefit and tended to increase fat-free mass. This
study provides important information for decision
making in clinical practice. Further research
should investigate the longer term effectiveness
of these agents.
The use of bioelectrical impedance is a
limitation to this study. The study was conducted
in many centres where the use of other body
composition methodology such as dual energy X-ray
absorptiometry was not possible. The prediction
equations used in this study were developed and
validated in HIV-positive subjects for both
cross-sectional and longitudinal measures [14],
and bioelectrical impedance analysis (BIA) is now
commonly used in the assessment of body
composition changes in studies of this nature
[16,33].
...No
significant differences were detected between the
placebo and nandrolone groups for changes in serum
cholesterol (total, LDL or HDL), triglycerides,
glucose and insulin in the present study, although
a significant difference did exist between the
nandrolone and testosterone groups for LDL and
total cholesterol. These tests should be viewed in
the context of multiple post-hoc comparisons.
Nevertheless, caution is needed in using these
agents in HIV-positive patients with pretreatment
elevations in lipids and poor glycaemic control,
as use in these populations remains
untested..."
INTRODUCTION
Despite
the introduction of highly active antiretroviral
therapy (HAART), weight loss continues to be
prevalent in HIV disease [1,2]. More importantly,
the introduction of HAART has not been associated
with increases in the fat-free mass [3], the body
composition component associated with survival in
HIV disease progression [4]. Reduced energy intake
has been shown to be the primary determinant of
weight loss in HIV disease [5]; however, other
metabolic, psychological, endocrinological and
gastrointestinal factors may contribute to
negative energy balance [6]. Lowered testosterone
levels have also been associated with loss of
fat-free mass in HIV disease [7].
There are a number
of different pharmacological agents available to
assist in management of HIV-associated weight loss
[8]. These agents include appetite stimulants such
as corticosteroids, megestrol acetate, thalidomide
and anabolic and androgenic agents (e.g. growth
hormone, nandrolone decanoate and testosterone).
The anabolic and androgenic agents are the only
ones to have shown consistent improvements in both
weight and fat-free mass [9-12].
Currently, in the
clinical management of weight loss in HIV-infected
patients, there remains uncertainty about the best
approach. After investigation of any underlying
cause of weight loss, such as inadequate dietary
intake, diarrhoea, opportunistic infections or
malignancy, there are no clear guidelines as to
the most appropriate agent to use to assist
patients to increase fat free mass.
There were two
primary aims of this study. First, we intended to
compare the effectiveness of 12 weeks of therapy
with nandrolone decanoate with placebo in
HIV-positive men with mild to moderate wasting on
fat-free mass. Secondly, we intended to compare
nandrolone decanoate and testosterone in order to
provide data for clinicians for decision making on
the most effective agent for increasing fat-free
mass. Secondary analyses compared changes in
weight, body fat, intracellular and extracellular
water, body cell mass, perception of treatment,
HIV disease markers and safety between nandrolone
decanoate and placebo or testosterone.
RESULTS (tables
immediately follow the text below, followed by
author Discussion & Methods)
From January 2000 to October 2001, 303
subjects from 21 sites in 11 countries were
recruited and randomized. All subjects commenced
treatment. Of the 157 patients randomized to
nandrolone, 152 completed the 12-week treatment
(97%), of the 66 randomized to testosterone, 59
completed the treatment (89%), and of the 80
randomized to placebo, 77 completed (96%) the
12-week study period. Flow through the study is
outlined in Fig. 1. Seven subjects discontinued
because of adverse events, five from the
testestorone arm and one each from the nandrolone
and placebo arms. In two cases of adverse events
related to study withdrawal from the testosterone
arm (one subject withdrew because of buttock pain
on injection and one because of ongoing weight
increase), the cause was likely to be related to
the study drug. Depression as an adverse event
resulted in study termination in two subjects (one
in the nandrolone arm and one in the placebo arm)
and was thought to be unrelated to the study
medication. Three other subjects in the
testosterone arm experienced adverse events
resulting in study termination which were thought
to be unrelated to the study medication (rash in
one patient, pain not otherwise specified,
dehydration, diarrhoea, pyrexia and mouth
ulceration in one patient, and respiratory
insufficiency in the other patient).
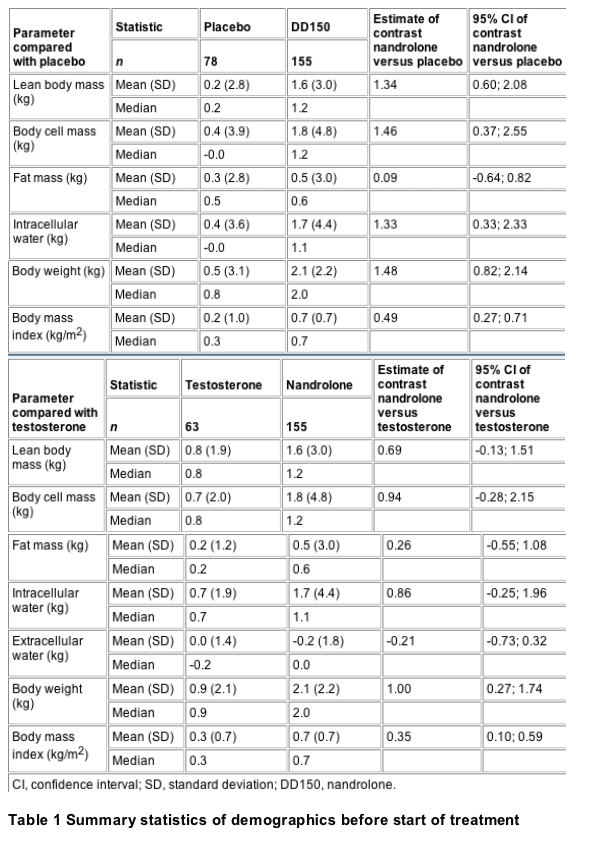
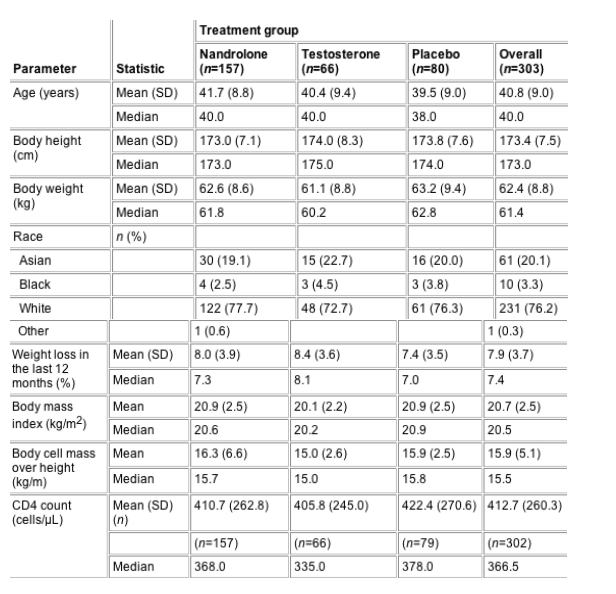
Baseline
demographics for each of the groups and the whole
study population are shown in Table 1. Changes
over time for the body composition parameters for
nandrolone versus placebo are presented in Table
2; the analysis presented in this table was
performed on an intention-to-treat basis. When
analysed on a per protocol basis, the changes of
fat-free mass in the nandrolone group (mean
increase 1.71 kg; n=145) were significantly
greater than in the placebo group (mean increase
0.32 kg; n=72; P=0.0007); the estimated mean
contrast was 1.38 kg [95% confidence interval (CI)
0.59; 2.17]. Table 2 also shows the contrasts and
confidence intervals for the differences in the
changes in body composition variables between the
nandrolone and testosterone groups.
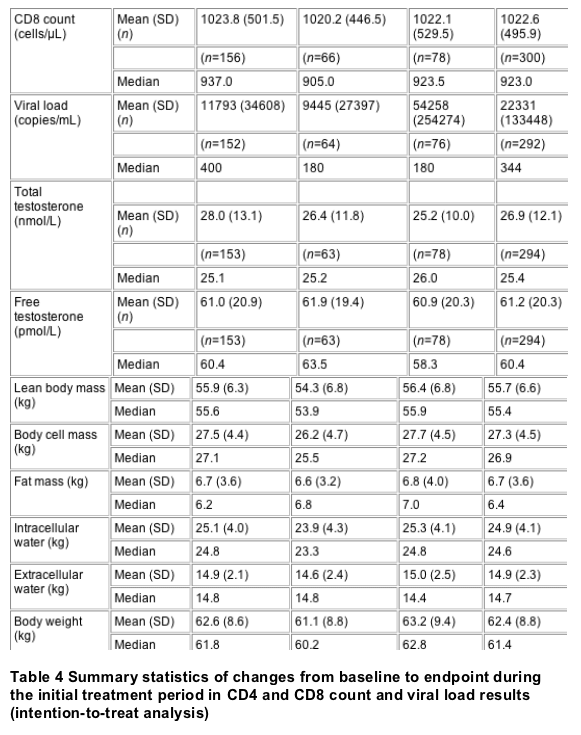
Table 3 shows the
results for the two BACRI domains. Table 4
presents summary statistics of changes from
baseline to endpoint during the initial treatment
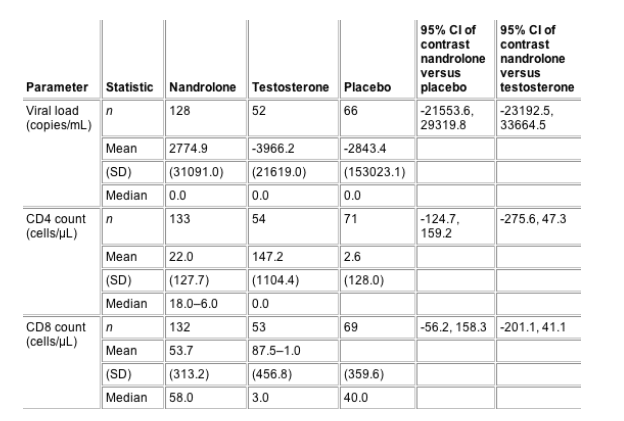
period in CD4 and CD8 count and viral load for the
intention-to-treat group. Considering the large
variability in these immunological parameters, no
relevant differences were noted. Changes in total
(P=0.024) and LDL (P=0.038) cholesterol were
significantly different among the three groups.
Post hoc analysis showed that the change in
cholesterol was significantly different between
the nandrolone decanoate group (change of
0.346±0.879 mmol/L from baseline) and the
testosterone group (change of -0.105±0.959 mmol/L
from baseline, P=0.005); the change in the placebo
group (0.348±2.027 mmol/L) was not significantly
different from that in the other two groups. The
post hoc analysis for LDL cholesterol similarly
showed a significant difference between the
nandrolone (change of 0.241±0.827 mmol/L) and
testosterone groups (-0.171±0.899, P=0.010), with
the change in the placebo group (0.058±0.958
mmol/L) being not significantly different from
that in the other two groups. Changes in HDL
cholesterol (P=0.318), triglycerides (P=0.266),
glucose (P=0.109), insulin (P=0.828), alanine
aminotransferase (ALT) (P=0.208), aspartate
aminotransferase (AST) (P=0.289), bilirubin
(P=0.862) and PSA (P=0.599) were not significantly
different among the three groups. The interaction
term between baseline testosterone quartile and
treatment was not significant for any of the
testosterone categories (P>0.214). Therefore
there is no testosterone level for which
nandrolone treatment would be superior to
testosterone or vice versa.
Table 2 Summary statistics of the
change from baseline to endpoint of the initial
treatment period of other body composition
parameters, body weight and body mass index
(intention-to-treat analysis)
Discussion
This study is the
first large multicentre randomized
placebo-controlled study to demonstrate the
effectiveness of nandrolone decanoate over placebo
in increasing fat free mass, weight and body mass
index in HIV-positive males with wasting. In
addition, fortnightly treatment with 150 mg
nandrolone decanoate was superior to fortnightly
treatment with 250 mg testosterone for increasing
weight, increasing body mass index and
(insignificantly) increasing fat-free mass.
Subjectively, nandrolone was superior to both
placebo and testosterone for improving perception
of treatment benefit and recovery from
symptoms.
Testosterone and nandrolone were chosen
as the two agents to compare in this study as they
were the treatments most commonly prescribed at
the participating research centres for treatment
of weight loss. Growth hormone has also been
shown, in two large randomized placebo-controlled
studies [9,12] and in a single-arm study [16], to
increase weight and fat free mass in people with
HIV-associated wasting [9]. However, the option of
using growth hormone is not available in many
countries where high cost, difficulty with daily
injections, issues of injection equipment safety
with home injection and side effects all limit its
current and potential usefulness. In view of the
escalating nature of health care costs, it is
critical that an effective anabolic agent that
will improve fat free mass and perception of
treatment benefit is of low cost and has minimal
side effects so that it may be of use in
resource-poor settings.
The initial randomized controlled trial
of growth hormone for HIV-associated wasting
reported a change in weight of 1.1±4.0 kg
(P<0.0001 compared with placebo;
intention-to-treat) and a change in fat-free mass
of 2.5±3.4 kg (P<0.001 compared with placebo;
intention-to-treat) [9]. The more recent trial
showed weight increases of 1.5 or 2.2 kg (dose
dependent) (P<0.0001) with increases in fat
free mass of 3.3 and 5.3 kg (P<0.0001) versus
placebo [12]. The changes in weight in the present
study are similar to those found in this previous
research, although the fat-free mass change was
greater with growth hormone. Differences in entry
criteria and patient demographics preclude a
direct comparison of the results of this study and
the growth hormone research; however, the economic
costs of the treatment cannot be overlooked in the
current health care environment. A significantly
greater proportion of patients receiving growth
hormone reported swelling/puffiness,
arthralgia/myalgia and diarrhoea compared with the
placebo group. In the most recent study,
49.8-51.8% of those receiving growth hormone
reported adverse events including arthralgia,
myalgia, peripheral oedema, hypothesia and
paraesthesia compared with 28.7% of the placebo
group. These side effects must be considered when
making treatment decisions.
The results of the present study support
previous research prior to [10,17] and since
[18,19] the introduction of highly active
antiretroviral therapy which demonstrated
significant within-group changes in weight and fat
free mass in HIV-positive male subjects receiving
nandrolone in open-label studies. These previous
studies reported sample sizes of between eight and
16 subjects, with doses varying from 100
mg/fortnight to an average of 550 mg/week for
periods between 10 and 16 weeks. Three studies
investigated the effects of nandrolone in
weight-losing subjects [10,17,18] and one in
weight-stable subjects [19]. Strawford et al [10]
specifically recruited hypogonadal subjects.
Testosterone levels were within the normal range
in one study [19] and not measured in two studies
[17,18]. Virological control and CD4 levels and
methods of assessing body composition differed in
these previous studies. Within-group weight
increases in these studies were between 2 and 5 kg
and fat free mass increases were between 3 and 4
kg.
It is of
particular interest that the subjects in the
highest dose study [19] did not show greater
weight and fat free mass increases than those
found in the other published studies, despite
using a substantially higher dose of nandrolone.
Subjects in this high-dose study were weight
stable and eugonadal, and this suggests that
nandrolone may be more effective in hypogonadal
and/or weight-losing subjects. This contradicts
the idea of the dose-response curve proposed by
Forbes [20], which suggests that responses to
pharmacological doses of anabolic agents are
greater than those to replacement doses.
The within-group
weight change in the present study was 2.1±2.2 kg
(P<0.0001 versus placebo) and that in fat free
mass was 1.6±3.0 kg (P=0.0006 versus placebo). It
is likely that the smaller changes in the present
study are a direct result of the large randomized
sample size including a diverse range of subjects
in terms of pre-entry weight loss, testosterone
status, disease stage and ethnic background.
A recent
meta-analysis of the effects of testosterone
versus placebo on fat-free mass showed a
significant mean increase in the testosterone
group of 0.51 kg (95% CI 0.09; 0.93) for the fixed
effects model and 1.22 kg (95% CI 0.23; 2.22) for
the random effects model [21]. The same analysis
showed positive weight increases of 0.63 kg (95%
CI-0.01; 1.28) for the fixed effects model and
1.04 kg (95% CI-0.01; 2.10) for the random effects
model. The results of the present study are
consistent with those of this meta-analysis,
although heterogeneity in dose, routes of
administration, and patient characteristics, such
as hypogonal versus eugonadal and weight-losing
versus weight-stable, makes a direct comparison of
studies difficult.
Rietschel et al [22] reported that 21%
of their HIV-positive male subjects using HAART
had lower than normal free testosterone levels,
suggesting that hypogonadism remains common in
HIV-positive male subjects taking HAART. Prior to
the introduction of HAART, this same research
group reported lowered free testosterone levels in
49% of a sample of HIV-positive male patients with
weight loss [7]. In the present study, 17% of
subjects (n=50) had low free testosterone (<42
pmol/L) and 5% (n=15) had low total testosterone
(<12.1 nmol/L). We would perhaps have expected
this prevalence to be higher based on the previous
literature and the bias in our subject group. As
the previous literature reports a relationship
between loss of fat free mass and lower
testosterone levels, we would have expected a high
prevalence of hypotestosteronaemia in this
sample.
It has
been reported that the lower than normal
testosterone level in HIV-positive male patients
is associated with decreased sexual functioning,
depression and loss of fat free mass [23,24]. The
aetiology of this physiological observation is
unclear. HIV may have an impact on the
hypothalamic pituitary axis or a direct impact on
the testis, or both. In addition to involuntary
weight loss, lowered testosterone levels are
associated with a poor prognosis [25]. Because of
these documented associations, the relationship
between baseline androgen levels and treatment
with androgenic anabolic agents warrants
investigation. Bhasin et al [26] did not find a
correlation between change in fat free mass and
baseline testosterone levels in their study
investigating the effects of testosterone
replacement in hypogonadal HIV-positive men with
weight loss. In the present study, we aimed to
determine whether one of the treatments would be
superior in increasing fat free mass at different
testosterone levels. The testosterone-by-treatment
interaction term was not significant, revealing
important clinical information that neither
treatment is superior to the other in increasing
fat free mass at lower or higher testosterone
levels.
This
study also addresses the concern that
anabolic/androgenic agents may increase serum
lipids and liver function test results, and have a
negative effect on glycaemic control [27,28].
Current evidence does not support these concerns.
Replacement doses of testosterone in middle-aged
men without HIV infection have been associated
with reduced visceral adipose tissue, lower
glucose levels and improved insulin sensitivity
[29]. Epidemiological studies have shown inverse
relationships between total and free serum
testosterone and visceral fat, cardiovascular
disease and type 2 diabetes [29]. Sattler et al
[30] demonstrated no detrimental effects of
nandrolone on triglycerides, or total or LDL
cholesterol. HDL cholesterol did decrease
transiently during nandrolone treatment, but
returned to near-baseline levels when assessed 12
weeks after the treatment was finished. Previously
we have also investigated changes in serum lipids,
fasting insulin and glucose over an 8-week period
in 10 HIV-positive male patients with body
composition changes consistent with lipodystrophy
and found no significant changes in these
parameters [31]. No significant differences were
detected between the placebo and nandrolone groups
for changes in serum cholesterol (total, LDL or
HDL), triglycerides, glucose and insulin in the
present study, although a significant difference
did exist between the nandrolone and testosterone
groups for LDL and total cholesterol. These tests
should be viewed in the context of multiple
post-hoc comparisons. Nevertheless, caution is
needed in using these agents in HIV-positive
patients with pretreatment elevations in lipids
and poor glycaemic control, as use in these
populations remains untested.
27 Glazer G.
Artherogenic effects of anabolic steroids on serum
lipid levels. A literature review. Arch Intern Med
1991; 151: 1925-1933.
28 Kopera H. (1993) Side effects and
contraindications of anabolic steroids, In: Kopera
H. ed. Anabolic-Androgenic Steroids Towards the
Year 2000, pp. 262-266. Vienna, Blackwell MZV.
29 Bhasin S. Effects
of testosterone administration on fat
distribution, insulin sensitivity, and
atherosclerosis progression. Clin Infect Dis 2003;
37 (Suppl. 2): S142-S149.
30 Sattler FR, Schroeder ET, Dube MP et
al. Metabolic effects of nandrolone decanoate and
resistance training in men with HIV. Am J Physiol
Endocrinol Metab 2002; 283: E1214-E1222.
31 Gold J, Batterham
M Nandrolone decanoate; use in HIV-associated
lipodystrophy syndrome: a pilot study. Int J STD
AIDS 1999; 10: 558.
Adverse events thought to be related to
the study medication were rare and occurred
primarily in the testosterone group, although the
differences between groups were not statistically
significant.
The
BACRI was specifically designed and validated to
show subjective recovery from the symptoms of
anorexia/cachexia and patient perception of
benefit with nutritional interventions [15]. The
instrument was initially tested in a dose-ranging
randomized placebo-controlled trial of megestrol
acetate for HIV-associated wasting [32]. In this
study, the BACRI was reported not only to
differentiate positive effects of treatment versus
placebo but also to differentiate dose effects
[15]. The use of this instrument in the present
study clearly demonstrated a significant benefit
of nandrolone over both placebo and testosterone
for subjective improvements in recovery of
symptoms from anorexia/cachexia and patient
perception of benefit. This questionnaire was
designed to target the aspects of quality of life
that are specific to a nutritional/appetite
intervention, such as body image, enjoyment of
food, appetite per se and the actual intervention.
These areas are not covered in traditional quality
of life questionnaires and therefore a treatment
effect may be missed.
Clinically, this study demonstrates
unequivocally the superiority of nandrolone
decanoate over placebo for increasing fat-free
mass, weight, body mass index, and perception of
treatment benefit when compared with placebo. In
addition, nandrolone was superior to testosterone
for increasing weight and perception of treatment
benefit and tended to increase fat-free mass. This
study provides important information for decision
making in clinical practice. Further research
should investigate the longer term effectiveness
of these agents.
METHODS
Subjects
Potential
subjects were recruited through contact with
clinicians and multidisciplinary HIV health care
workers. To participate, subjects had to be
HIV-positive male adults with mild/moderate
wasting and an energy intake greater than 75% of
requirements, estimated using the Harris &
Benedict equation [13] by a 24-h dietary recall,
on stable antiretroviral therapy including at
least two agents, with a CD4 count >50
cells/_L. For this study, mild/moderate wasting
was defined as a weight loss of 5-15% in the last
12 months, or a body mass index of 17-19 kg/m2, or
a body cell mass/height ratio <13.5 kg/m (13.5
kg/m was defined as the 5th percentile of the
normal population based on 43 measures in
HIV-positive weight-stable patients). Exclusion
criteria included a weight increase of >3% in
the last 2 months; hypersensitivity to
anabolic/androgenic agents; prostate or mammary
cancer; use of anabolic/appetite-stimulating
agents in the last 3 months; chronic use of
systemic corticosteroids (except topically);
significant cardiac, renal, hepatic or other
disease which may prevent study completion; an
AIDS-defining illness (other than HIV wasting
syndrome) within the last 2 months; malignancy
other than Kaposi's Sarcoma localized to the skin;
involvement in a vigorous resistance exercise
training programme (weight training) in the last 2
months; liver function tests (aspartate
transaminase/alanine transaminase) five times the
upper limit of normal; fasting total cholesterol,
triglycerides, insulin or serum creatinine≥three
times the upper limit of normal; platelets≦50 000
cells/_L; haemoglobin≦8.0 g/dL; prostate specific
antigen (PSA)≥4 ng/mL; active drug abuse; alcohol
consumption>four standard drinks/day;
administration of any investigational drug in the
last 3 months. Each participant gave written
informed consent and institutional ethics approval
was obtained at each study site.
Protocol
Eligible subjects were randomized per
site, with each consecutive subject recruited to
receive the next study identification number from
lowest to highest. For emergencies, a drug
identification record was supplied to the
investigator at each site. The randomization was
weighted 2:1:1 with 150 subjects to receive 150 mg
nandrolone decanoate, 75 to receive 250 mg
testosterone (30 mg propionate, 60 mg
phenylpropionate and 100 mg decanoate) and 75 to
receive placebo. Each treatment was given as a
1-mL oil-based intramuscular injection in the
gluteal region every fortnight. All injections
were given by qualified clinical staff and
compliance was confirmed by the study out-patient
records and vial count. The treatment period was
12 weeks.
Clinical endpoints
The primary endpoint was a comparison of
absolute change in fat free mass at 12 weeks
between the nandrolone decanoate and placebo
groups, and between the nandrolone decanoate and
testosterone groups. Secondary outcome measures
included change in weight, body fat, intracellular
and extracellular water, body cell mass, muscle
strength, perception of treatment, HIV disease
markers and safety.
Body composition
Age, body weight and height were
recorded. Body composition was assessed using
bioelectrical impedance analysis (RJL101; RJL
Systems Inc., Clinton Township, MI, USA). Fat free
mass was assessed using the published equation of
Kotler et al [14], which has been validated for
use in HIV-positive subjects. Body composition was
measured at baseline, week 6 and week 12. Body
cell mass, intracellular and extracellular water
and fat mass were also calculated using validated
and published equations from the same source.
Perception of
treatment
The Bristol-Myers
Anorexia Cachexia Recovery Instrument (BACRI)
[15], an eight-item questionnaire, was used to
examine change in subjective recovery from
symptoms of anorexia/cachexia (seven items,
considered together as BACRI 7) and patient
perception of benefit (one item, termed BACRI 1).
BACRI 1 scores are measured on a scale ranging
from 0 to 10, where 10 denotes the largest
improvement. BACRI 7 scores are measured on a
scale of 0-70, where 70 denotes the largest
improvement. The BACRI was administered at week
12.
Laboratory
parameters
Laboratory parameters
were measured in a fasting state at baseline and
week 12. Liver function tests included ALAT/SGPT,
ASAT/SGOT, bilirubin and creatinine. Standard
biochemistry included total protein, albumin,
haemoglobin, leucocyte count and platelet count.
Cholesterol [total, high-density lipoprotein (HDL)
and low-density lipoprotein (LDL)], glucose,
insulin and glycosylated haemoglobin (HbA1c) were
measured as markers of lipid and glucose
metabolism. CD4 T-cell count and HIV RNA viral
load were measured as markers of HIV disease
progression. Prostate-specific antigen (PSA) was
also assessed. All sites conducted their own
laboratory investigations.
Data analysis
Data
were analysed using SAS version 8 or higher (SAS
Institute, Cary, NC) using the general linear
model (proc glm). An analysis of variance was
applied with change from baseline to 12 weeks in
fat-free mass as the dependent variable. Treatment
and centre were entered as factors. Interaction
between treatment and centre was investigated.
Confidence intervals and estimated means for the
contrasts of nandrolone decanoate versus placebo
and nandrolone decanoate versus testosterone and
summary statistics are presented. Analysis was
performed on an intention-to-treat basis with the
last observation carried forward. In order to be
included in the intention-to-treat analysis,
subjects must have had at least one post-baseline
fat free mass assessment. Differences between
groups in changes in serum lipids, glucose,
insulin and PSA were assessed using nonparametric
statistics. The superiority of nandrolone or
testosterone for eliciting an increase in fat-free
mass at various levels of baseline testosterone
was investigated by incorporating an additional
interaction term (baseline free or total
testosterone level in halves, tertiles, quartiles
or quintiles by treatment group) into the general
linear model discussed above.
Author
Affiliations
1The Albion Street
Centre, Surry Hills, NSW, Australia, 2The
University of Wollongong, Wollongong, NSW,
Australia, 3Organon International, Oss, The
Netherlands, 4Infectious Diseases Service, State
Servant Public Hospital, Sao Paulo, Brazil,
5Institute of Infectology Emilio Ribas, Sao Paulo,
Brazil, 62nd Internal Medicine Clinic, 1st IKA
Hospital, Melissia, Greece, 7Department of
Endocrinology, Cisanello Hospital, Pisa, Italy,
8Division of Allergy and Clinical Immunology,
Department of Clinical Medicine, University of
Rome "La Sapienza", Rome, Italy, 9Department of
Medicine, Hospital Kuala Lumpur, Kuala Lumpur,
Malaysia, 10AIDS Diagnosis and Therapy Center,
Warsaw, Poland, 11Infectious Diseases and AIDS
Unit, Hospital Clinic, Barcelona, Spain, 12Thai
Red Cross AIDS Research Center, Chulalongkorn
Hospital, Bangkok, Thailand, 13Bamrasnaradura
Hospital, Nonthaburi, Thailand, 14Chelsea &
Westminster Hospital, London, UK, 15Holdsworth
House, Darlinghurst, NSW, Australia and 16Academic
Medical Center, Amsterdam, The Netherlands
|
|
| |
| | | | |
| |
| | |